new web: http://bdml.stanford.edu/pmwiki
TWiki > RisePrivate Web>DryAdhesion? >StanfordDryAdhesives (10 Oct 2005, MarkCutkosky)
RisePrivate Web>DryAdhesion? >StanfordDryAdhesives (10 Oct 2005, MarkCutkosky)
Thinking of trying some filters made of mixed cellulose (from Millipore), which would be very easy to etch away without harming the urethane. However, these do not have nice round pores, they have more of a sponge-like structure. So they wouldn't leave hairs, but rather some sort of branched structure. This may not be ideal for adhesion, but if it results in a highly compliant structure it could be a good backing structure on which to mount the adhesive structure. i.e. a structure with lamellar-type properties in that it would provide a higher level of compliance.
update: ordered mixed cellulose filters from Millipore, http://www.millipore.com/catalogue.nsf/docs/SSWP01300.
Using PVA as release coating on filters: Peel filters off? -- MiguelPiedrahita? - 02 May 2005 Possibility of peeling off filter rather than etching off. This process is mentioned in several papers, including "Synthetic gecko foot-hair micro/nano-structures as dry adhesives", Journal of Adhesion Science and Technology, Sitti & Fearing. Contacted Rick to get details of process, because apparently the filter has to be initially bonded to a substrate so that it can be peeled off later. This is confirmed by trying to peel filter off an existing urethane sample - the filter refuses to come off the sample. Response from Rick:
If you're refering to the structures I think your are, then
1) they are several microns wide and only 8 microns tall (very low aspect ratio).
2) the bumps (I hesitate to call them hairs or fibers) are made of PDMS. PDMS does not bond to other stuff so well, plus it has low stiffness, high elastic limit, incompressible. So when pulled out of the mold, the PDMS bumps neck and release. This doesn't work so well with the combinations of polyurethanes, epoxies, and polyimides and filters (with high aspect ratios) that we've tried. You could make pdms hairs, but then there's some question as to why you don't just use a flat pdms surface (which gives more adhesion than a structured pdms surface). When you try to pull a stiffer polymer out of the filter, the hairs generally rip off, or the filter just doesn't come off. I believe they were "bonding to the substrate" by putting the filter on a piece of gel-pak. A layer of polymer is applied to top of the filter and the filter is vaccuum filled. The gelpack side is the free side of the fibers. 04 May 2005 --- One possibility to get around the problem of etching polycarbonate without harming urethane is to peel the filter off instead of etching. One approach that Rick has tried that shows some promise it to use a PVA release coating on the filter. PVA is etched in water, and then the filter can be easily removed.
Dipping alone will completely clog the pores. Can use a small fixture that holds the filter around the edge. Cover the filter with PVA and then force air through to open up the pores. Even using this process, 2um pores give considerably smaller diameter fibers.
Other techniques for depositing PVA may work, (e.g. vapor deposition, dilute solution, etc.) may work but haven't been developed yet.
20 May 2005 --- Immersed filters in PVA to try approach described below (04 May). Tried 100% PVA, as well as solutions of 25% and 50% PVA. Filters were immersed for a few seconds and then allowed to air dry. After drying, the filters were examined under 800x magnification. Pores were still clearly visible on all filters, but it was impossible to tell if pores are clogged or not. May just have to try casting and see what happens.
9 June 2005 --- Immersed filters in PVA, allowed to air dry, then cast on thin film of urethane. Inspected at 800X, pores could be very clearly seen as dark blue rings. Blue color likely from PVA, which is blue. Immersed filter in water for ~5 minutes to dissolve PVA, and tried to physically peel filter off. Filter is very securely bonded to urethane film and refuses to peel off.
-- MiguelPiedrahita? - 17 May 2005
Met with Sangbae and Suk-Won Cha (post-doc in Fritz Prinz' lab). Suk-Won shared his ideas regarding the problem of fabricating hair-like micro-structures. The two main possibilities in his opinion:
- Casting into micro-pore filter:
- possibility of using alumina filters, which inherently have a somewhat hierarchical structure. Etch with KOH.
- Polymers must have a relatively low molecular weight; otherwise, the molecules are too large to fill the mold well for 0.2um pores. Should be less than 20,000 amu.
- One challenge with using a micro-pore filter is that we are restricted to castable materials. Is there a way to fill a micro-pore filter using other methods such as vapor deposition? Vapor deposition would result in the entrance to the pores getting clogged very quickly, long before the filter is fully filled. It is possible to deposit a single atomic layer at a time, which would result in the pores filling evenly and fully. However, this requires special equipement and is very slow (growth of 1 micron takes 3 days!).
- Growth of hairs using sputtering:
- From http://www.angstromsciences.com/technology/sputtering.htm: Sputtering is performed by applying a high voltage across a low-pressure gas (usually argon at about 5 millitorr) to create a "plasma," which consists of electrons and gas ions in a high-energy state. Energized plasma ions strike a "target," composed of the desired coating material, and cause atoms from that target to be ejected with enough energy to travel to, and bond with, the substrate.
- Variations include using evaporation or laser ablation to cause atoms from target to be ejected towards substrate.
- To grow hair-like structures, the substrate can be angled with respect to the target ("oblique angle deposition"). The substrate is then rotated during deposition, so that the "hairs" are actually spring-shaped. The rate of rotation can be changed throughout the deposition process to create some changes in the geometry of the hair. There is plenty of literature in this area.
- Most materials can be sputtered, including polymers (which may need an RF (AC) voltage instead of DC).
- growth rates are on the order of 1 micron/hour.
Coefficient of thermal expansion for polycarbonate: 6.5 x 10-5 cm/cm/°C
Coefficient of thermal expansion for polyurethane: 10 x 10-5 cm/cm/°C
silicone Tried casting 2.0um filters with silicone. When cured, filter peeled easily off silicone. Inspection under 800x microscope revealed very irregular, pitted surface under area where filter was. Hairs were probably pulled off with filter. Pits could be tiny bubbles and/or root of hairs. Sample wasn't degassed properly because vacuum pump has been in use by another project for the past few days. May try again with proper degassing.
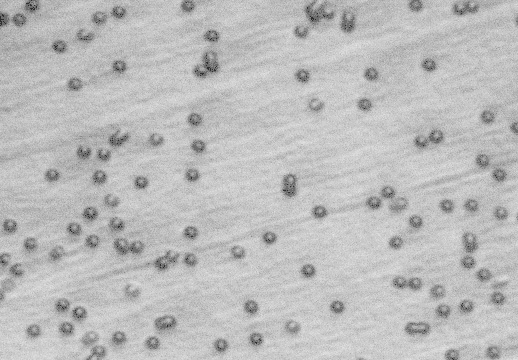
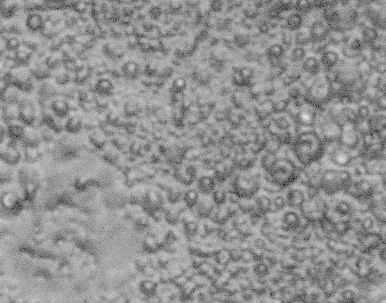
Solvents Apr. 22 2005. We made several samples using 2.0um pore filters with urethane. Upon etching with methylene chloride, it became apparent that urethane is attacked by methylene chloride. Thin films of urethane instantly shriveled up upon contact with the solvent. We examined samples (pre and post-etching) under a microscope at 800x magnification. Pre-etching, it appears that the filter disc was infiltrated by urethane quite well, and the pores are quite visible. Post-etching shows significant surface damage, both in areas where the filter disc was and on the bare urethane surface. See pictures below. It seems that this solvent will not work for this process.
- 2.0 um filter infiltrated with urethane:
- urethane after etching away filter:
- Plain urethane at 800x:

- Plain urethane after methylene chloride at 800x:

- Calcium hydroxide: Severe effect on polycarbonate, no effect on polyurethane. Also known as hydrated lime. Calcium hydroxide is a colorless or white powder, is slightly soluble in water to form "limewater".
- Hexane: Severe effect on polycarbonate, "fair" compatibility with urethane.
- Isopropyl ether: Severe effect on polycarbonate, "fair" compatibility with urethane.
- Lye: Severe effect on polycarbonate, "fair" compatibility with urethane.
- Methyl Acetate: Severe effect on polycarbonate, "fair" compatibility with urethane.
Suppliers of filters of different materials:
- http://www.m-techdiagnostics.ltd.uk/membranes.shtml
- http://www.whatman.com/products/?pageID=7.57.291
- http://www.millipore.com/catalogue.nsf/webvirtual?open&search_criteria=cat_id=5CKHLL
-- MiguelPiedrahita? - 15 Apr 2005 Got quote for polyimide used by Rick, $560 for 250g! From HD Microsystems.
Filter materials
Alumina Filters (info from Rick): The alumina filters tend to have a section that is somewhat thicker and mildly hierarchical in the last couple microns of the one side. We have moved away from alumina filters because of the limitation in pore sizes. Due to the fabrication process (anodizing aluminum under special conditions), porous alumina generally has a max pore size of about 0.3um diameter. The largest we typically find commercially are 0.2um. You can get very small pores, e.g. 0.05um. Also due to the process, the pores are very close together. For example, the 0.2um pores have center to center spacing of about 0.3um. We're talking to some people here who make porous alumina. Anyway, the resulting fibers were generally too closely packed for us. We lowered density somewhat by blocking pores, but we moved to polycarbonate because of the greater variety of geometries.
Notes from telecon (Miguel, Dana, Rick) on Apr 13 2005:
- Rick currently casts hairs from polyimide. Polyimide may not lend itself well to integration into a foot because it is fairly inert and may not bond well to polyurethane. Therefore, we should try the casting process using polyurethane. If successful, possible approach would then be to mold hairs at the same time that we cast the foot body itself, then etch the filter away.
- Can we get samples from Rick? Not very easily, because current process uses polyimide that is spun-coated on glass slides. It is difficult to scrape large sections off the glass. They have done some samples on PVA instead of glass, may be more promising, but it still needs work.
- Researcher working in Rick's group: possibility of custom polyurethanes with desired surface energy!
- Working on applying a monolayer to the hairs after casting to reduce clumping.
- SEM at Berkeley: samples no larger than a half-dollar. Samples are sputter-coated with gold/platinum.
- best performance is 0.6um hairs or smaller.
- 2.0um hair diameter is good for verifying process, can be easily seen at 500x.
- can wipe top of filter with q-tip with solvent to expose hairs in case filter isn't fully infiltrated.
- must lay filter on polymer layer gently and evenly.
- filter changes color when liquid touches the bottom, then again when it infiltrates through to the top. Infiltration generally takes tens of seconds.
- Can scrape a sample to push hairs over in order to see the hairs in the microscope.
- try 500x microscope again, make sure we can see 2um pores in filter ok.
- get polyimide! May need to spin-coat.
- try bonding polyurethane to polyimide.
- try process as described by Rick using polyimide in 2um filters, verify results under microscope.
- try process using polyurethane.
- send samples to Rick
- schedule visit to Berkeley after Rick has seen samples.
Quick experiment to conduct: Look for suitable combination of casting material, filter material, and solvent. We will initially try materials we are familiar with and have access to:
- Casting materials: Hard polyurethane, epoxy
- Filter material: Polycarbonate
- Solvents: Acetone, methylene chloride
Tested polyurethane, epoxy, and polycarbonate filters in acetone and methylene chloride.
Filter was not affected by acetone after 30min, thus acetone does not make a suitable solvent.
Filter dissolved in ~2 seconds in methylene chloride. Polyurethane and epoxy were softened by methylene chloride after a few minutes of soaking.
Conclusion: Polyurethane cast into polycarbonate filter could work well by dipping in methylene chloride for a few seconds to etch the filter without harming the polyurethane.
Information from Rick Groff
Email exchange between Moto and Rick (Dec.17, 2004): I haven't cast polyurethane over polyimide, so I don't know for sure, but I think it would work fine. I've used epoxy on polyimide and that worked well, but I recall you mentioned methylene chloride would damage epoxy. Correct? Yes, the the methylene chloride attacked the epoxy, but once again I think that was mostly a surface effect. A thin film would crack apart, but a big chunk would seem relatively unaffected. (The surface would mist up a little.) If you are dealing with methylene chloride already, would it be OK to have you send us some filled polycarbonate filters, have us cast polyurethane over it, and send them back to you for etching and observation? Let me know. This would be possible. Which polymer are you using. We bought a bunch of the different Innovative PUs, so we may well have the one you're using here. If we're just testing for a bond to a blob of PU, we could do the whole process here. If you're working on getting it to attach to some feature (which will probably be harder, to avoid PU getting onto the wrong side of the filter), that would be better done on your side. It would also be interesting to mold both the hair and skin/feet together by setting your filters in SDM molds. The challenge is how to pull vacuum. Maybe we can do the pouring in a vacuum chamber. Another option might be to apply high pressure from outside to push the material into the filter pores. We generally fill the filters using capillary action these days. The more difficuly problem is keeping them from overfilling. Filling at this point generally involves carefull timing to make sure the viscosity of the liquid polymer is right so that it stops at the right level.Email exchanges between Dana and Rick Groff (Jan 2005): Dana: How are you testing the bond between the hairs and a blob of polyurethane? Are you not worrying about getting polyurethane on the hairs? Which polyurethanes do you have? I guess we're trying to see which polyurethane blob the hairs bond well with first before we try attaching the hairs to a polyurethane feature (which I can definitely do), right? I just wanted to make sure we were on the same page. What are the results of your tests for bonding between the PU and hairs? Which PUs have you tried and which ones haven't you tried? If we still need to do more tests to find the right PU, could you send me a few samples so I can test them with some of our PUs? I realize that the chemicals you're using to etch the polycarbonate filters are toxic, but I think it would be useful to be able to etch the polycarbonate filters here. What chemicals are you using? I'll see if I can procure some. Rick: The filters we use are Millipore Isopores. http://www.millipore.com/catalogue.nsf/docs/C153 We use 13mm diameter filters. I'd suggest getting pore sizes 0.4, 0.6, 0.8 and 2.0um (The 2.0um should be readily visible optically. The smaller sizes are a little tricky.) We buy them through Fisher Scientific http://www.fishersci.com The etchant is methylene chloride (CH2Cl2? ) OSHA data here: http://www.osha.gov/SLTC/methylenechloride/ You should be able to get it from your favorite chemical supplier. Probably available on campus. MC is very volatile, so you'll probably want a sealed container to keep the etch bath in. (Gentle agitation is generally required to get all the polycarbonate off, so a sealed container will keep you from spilling while swirling it a bit. We use a capped glass bottle.) Dana: What kind of polyamide are you using to cast the hairs? I want to try and get my hands on some and try testing the bond between the two materials. Rick: We use HD Microsystems 2611 polyimide. (Note that polyimide and polyamide are different polymers.) With polyimide, the spin coat will be more critical. It's hard to get thick layers (more than 20um or so) of polyimide to cure properly.
Ideas, requests, problems regarding TWiki? Send feedback