Give it a twist: The Role of Torsional Mechanics in the Fight Against Heart Failure
Ventricular torsion is an important part of normal cardiac function that is significantly impaired following MI and in heart failure. Studies show that the torsional mechanics of the healthy heart serve two main functions.
- One is to provide a "wringing" motion that helps the ventricles empty more completely, and thus supports pump function.
- The other is to reduce ventricular wall stress during systolic shortening, which lowers oxygen demand and improves contractile efficiency.
When torsion is impaired, a resulting imbalance in transmural strains is observed. Such imbalance has been shown to be an important driver of remodeling as the ventricular wall is triggered to dilate due to unphysiological stress concentrations and increased oxygen demand.
Several studies have shown that current therapeutic interventions (such as cardiac resynchronization therapy or reconstructive surgeries) do not improve compromised LV torsional mechanics, suggesting that LV torsional mechanics may be difficult to restore once they are compromised. This motivates an approach targeted at intervening early after MI in order to preserve LV torsional mechanics before they are irreversibly compromised.
Research Rationale: Preserving LV Torsion as a Means of Improving Heart Function
In the mammalian heart, torsion results from the structural architecture of myocardial fibers: epicardial fibers (external layer) are oriented in a left-handed helix, midwall fibers are oriented circumferentially, and endocardial fibers (internal layer) are orientated in a right-handed helix (Fig. 2A). Contraction of the left-handed epicardial fibers adds to positive (left-handed) torsion, while contraction of the endocardial fibers adds to a negative (right-handed) torsion. The force balance is typically in favor of the epicardial fibers due to their increased lever arm, resulting in a “wringing motion” (Fig. 2B).
During systole, the ventricle twists to generate a net counterclockwise rotation of up to 12-15 degrees, aiding the heart to properly empty and eject blood. During diastole, the torsion is rapidly lost to generate a pressure gradient resulting in a suction effect, allowing the heart to expand and fill. Thus, twist during ejection predominantly deforms the subendocardial fiber matrix, resulting in storage of potential energy. Subsequent recoil of twist deformation occurs during diastolic relaxation and filling, and is associated with the release of restoring forces.
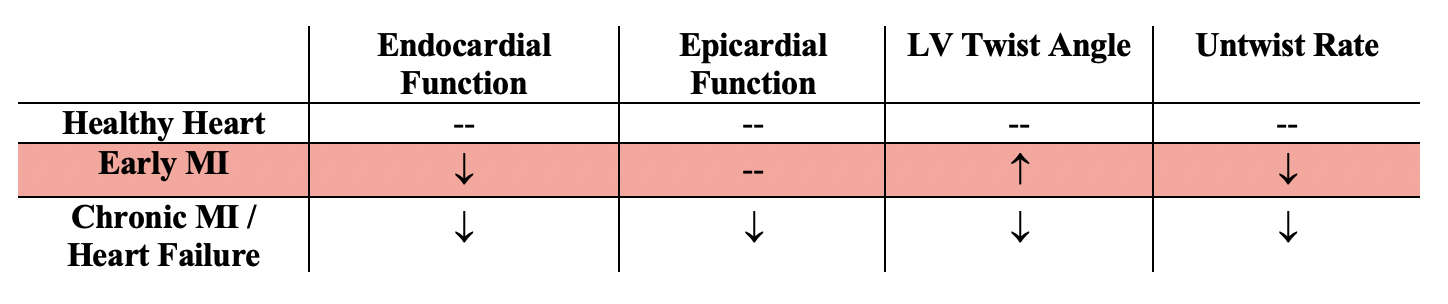
Studies have shown that when the balance of moments between endocardial and epicardial fibers is altered with changing loads and contractility, there is significant change in LV twist metrics. This explains altered LV twist dynamics observed in disease states. The majority of progressive myocardial diseases, including ischemia and early stages of myocardial infarction, tend to predominantly affect the endocardial layer. Thus, endocardial dysfunction has been shown to dominate and precede epicardial dysfunction. As a result, a transmural imbalance of torsional moments is incurred, affecting both the amplitude and the dynamics of twist. There are two metrics of interest when characterizing torsion: (1) LV twist angle, the degree of net twisting accomplished in contraction when comparing the heart’s base and apex, and (2) the untwisting rate, the velocity of elastic recoil during relaxation.
In early MI patients, initial depressed endocardial layer function leads to preserved or even increased LV twist angle as a result of the unopposed contraction of epicardial fibers. Ventricular relaxation, on the other hand, becomes abnormally slow (decrease in untwisting rate) as a direct result of endocardial dysfunction. This is because relaxation is affected by the “elastic spring” that is actively loaded during systole with the deformation of the endocardial layer. When this function is compromised, the storage and release of potential energy during systole, and commensurate elastic recoil during diastole, are immediately affected. In other words, if regional force generation is reduced in ischemia, the elastic “spring” is not fully loaded during systole, which decreases elastic recoil during relaxation. Eventually, with the onset of remodeling and compensatory mechanisms and deterioration of epicardial function, LV twist angle also becomes impaired, resulting in heart failure.
Metrics of interest are summarized in the table below (where “--" indicates baseline value):
Torsional mechanics are clearly essential in optimizing cardiac energetics and promoting pump function. Given the evidence that LV twist mechanics are difficult to restore once permanently impaired with the progression of heart failure, this proposal focuses on early intervention, motivating the following hypothesis:
It is hypothesized that preservation of LV torsional mechanics by means of external torsional support will shift cardiac energetics towards physiological ranges and assist in preventing the degeneration of ischemic heart disease to congestive heart failure
Overall, this work aims to lay the foundation for future research in the area of early, torsional support in prevention of heart failure. It does so proposing realistic means to implement such support mechanics and reporting results from computational simulations, prototyping, and preliminary bench tests.


