Category: MedicalRobotics
Clinical Problem: Congestive Heart Failure
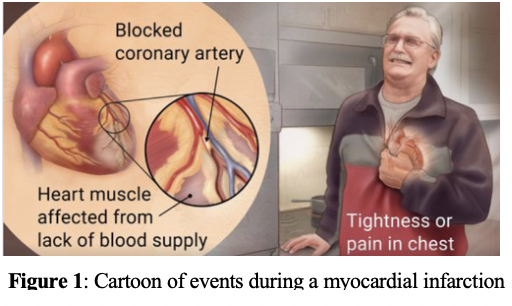
Coronary artery disease (CAD), a precursor to heart failure, is the leading cause of death in adults in the United States and in most developed countries. CAD can result in the formation of fibrous plaque in coronary arteries, reducing blood flow to the heart and decreasing oxygen supply to the tissues, known as ischemia. Persistent ischemia can induce myocardial cell death, known as a myocardial infarction (MI), or colloquially as a heart attack. Tissue death following CAD and MI, if left untreated, culminates into the heartís decreased ability to pump blood properly.
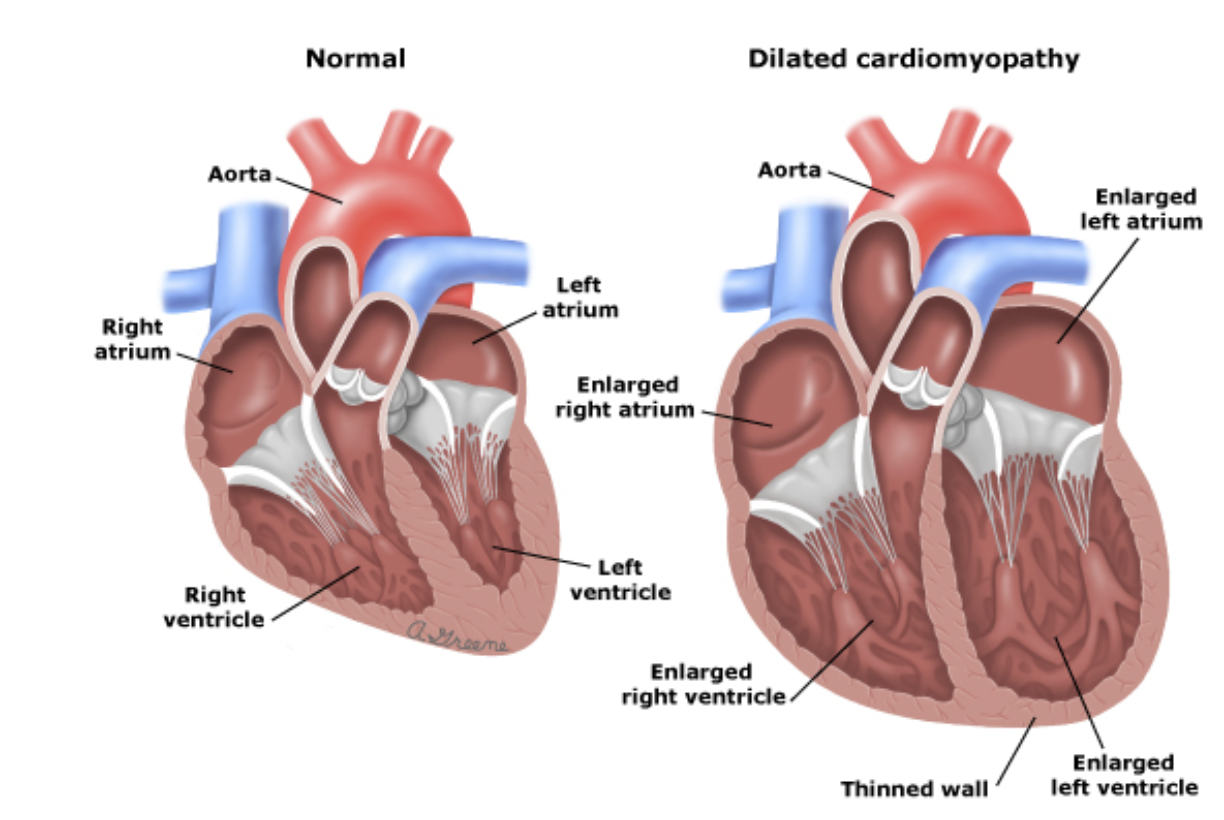
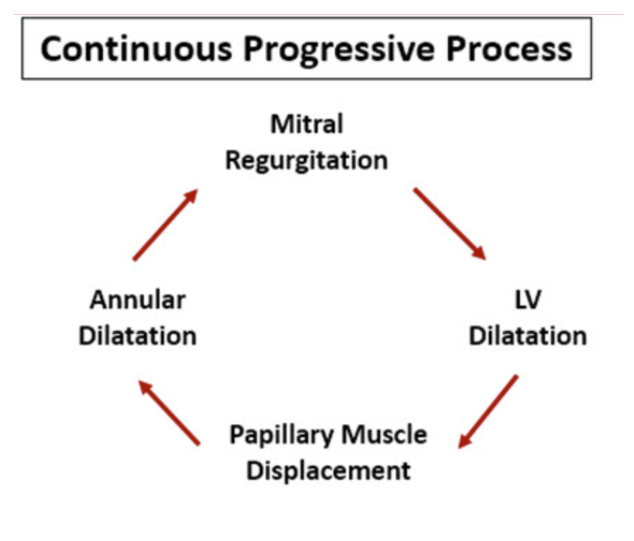
In order to compensate for its decreased pumping efficiency, the LV remodeling process involves short-term muscle hypertrophy, in an attempt to provide increased contractile function, followed by long-term progressive chamber dilation and wall thinning, as a result of increased chamber pressure and changes in the composition of the LV wall. The enlargement of the LV chamber leads to the migration of the papillary muscles, as well as dilation of the segment where the mitral annulus, or the structural component of the mitral valve, is housed. Such rearrangements, termed Dilated Cardiomyopathy, lead to impairment of mitral valve leaflet coaptation and give rise to mitral regurgitation, the backflow of blood.
Mitral regurgitation further exacerbates the heart's pumping efficiency, leading to further compensatory and maladaptive dilation of the LV. Thus, a positive feedback loop is triggered, and if left untreated, can spiral towards congestive heart failure and ultimately death. The purpose of the Cardiothoracic Surgical Devices Branch is to develop smart, mechanical interventions that mitigate or halt this destructive cycle.
Biomimetic Annuloplasty Ring for Mitral Regurgitation
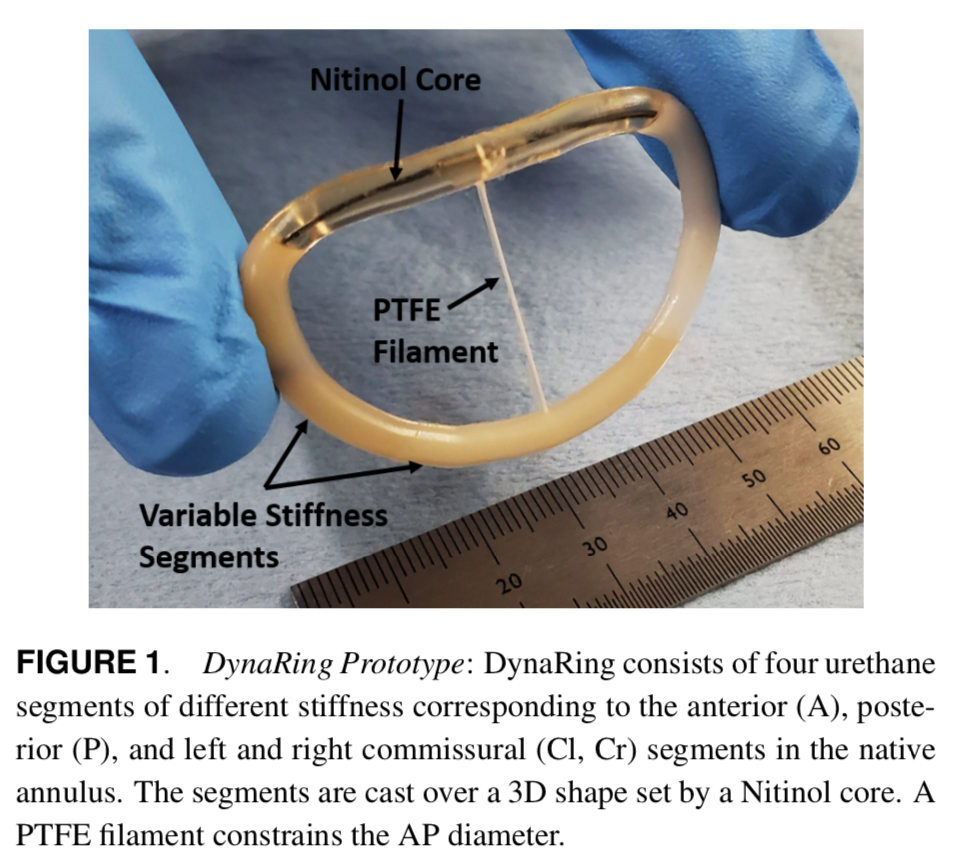
In this project, we explored the concept of a biomimetic annuloplasty ring to mitigate mitral regurgitation, a condition related and leading to heart failure. Building upon insights of the regional biomechanical properties of healthy mitral annuli, our work proposed the development of a selectively compliant annuloplasty ring, designed to match the material properties of the different segments of the annulus.
Overall, the project accomplished the following goals: (1) demonstrated the use of selective compliance in an annuloplasty ring to better enable mitral valve dynamics; (2) employed stereophotogrammetry to characterize and quantify the dynamic, cyclic motion of the prototype in a porcine mitral valve ex-vivo; and (3) established a standardized annuloplasty ring design process.
Early Post-MI Interventions to Mitigate and Prevent Progression of Heart Failure
MI leading to heart failure is a long, progressive, degenerative process, whereby both local and global mechanics of heart function are altered by adverse growth and remodeling in the surviving myocardium. Growth is defined as an increase in mass, while remodeling is defined as a change in shape or material properties, both of which can occur in tandem following an MI, eventually leading to heart failure.
Historically, mechanical solutions are designed to target late stages of heart failure following ventricular remodeling, when the left ventricle has significantly dilated, thinned and lost most of its function. Late-stage heart failure, however, has significantly been described as an elusive clinical target.Solutions aiming at passively restraining the dilated heart (i.e. ventricular restraint devices like CorCap and HeartNet) have not shown clinical significant or survival benefits. Solutions designed to actively aid the heart's pump function (i.e. LVADs), on the other hand, are associated with significant complications such as the risk of thrombosis and coagulation.
Such evidence highlights the necessity for mechanical solutions designed to be implemented earlier in the process, before structure and function are significantly compromised. At this point, most early-stage solutions are either pharmaceutical or biological and, while they can be effective at slowing down the development of symptoms, they are ineffective at preventing deleterious outcomes. This work is thus based on the premise that mechanically restoring or preserving cardiac function early in the process could help promote long-term survival and deter the progress of heart failure.
Several factors are important when considering the development of this complex disease, necessitating multifaceted solutions. In the pursuit of a comprehensive solution, we focus on two main components: (1) a torsional support device (TSD) investigating the feasibility of providing directional epicardial support in preservation of LV twist; and (2) a Biomimetic Reinforcement Project (BMR) aiming to develop local reconstructive solutions to compromised tissue.
Lab Members
Are you an undergraduate student looking to get involved in research? Are you a first-year PhD student interested in a rotation? Contact Ileana (ipirozzi@stanford.edu, lead on torsional support device) and Ali (akight@stanford.edu, lead on biomimetic reinforcement) to learn more about available projects!