Local Biomimetic Reinforcement
Can we optimize the heart?
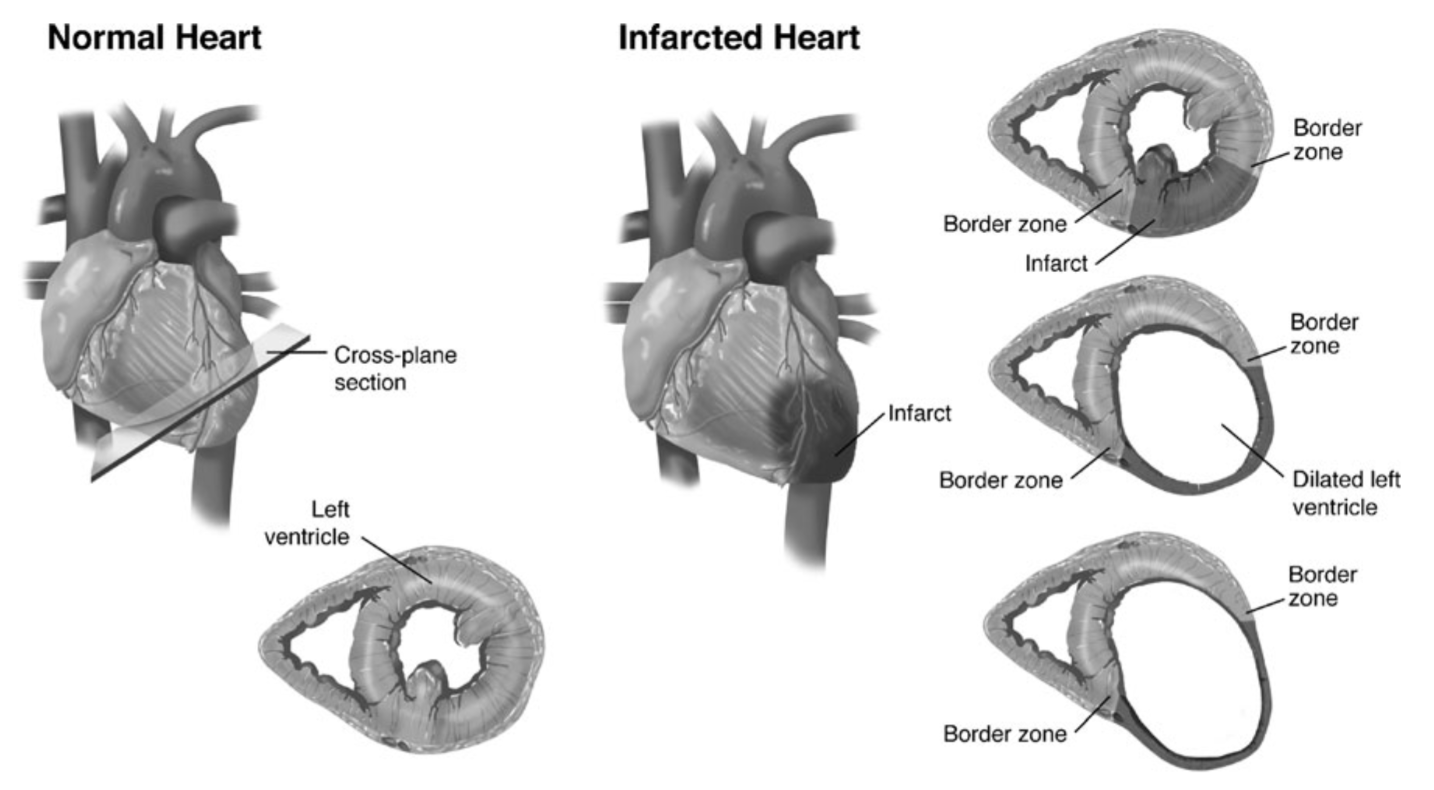
The intricate biomechanical architecture of the heart is fundamental to its function as the body’s engine, enduring over 3 billion beats in a lifetime. Complex three-dimensional fiber arrangement and temporal electro-mechanical coupling work in concert to sustain continuous blood flow and provide the necessary nutrients to organs and tissues throughout the body. The heart receives its own blood supply via the coronary arteries; when this supply is blocked due to plaque build-up from coronary artery disease, the heart experiences a lack of oxygen supply, often termed an acute myocardial infarction (AMI). AMI leads to local tissue death, also known as an infarct, resulting in the disruption of the heart’s precisely orchestrated structural function and triggering a destructive downhill progression towards heart failure.
Previous research on the biomechanical response to AMI has elucidated the importance of infarct expansion on the progression of heart failure. Infarct expansion is the immediate consequence of myocyte restructuring from tissue necrosis, consisting of the slippage and elongation of cardiac cells. In consequence, the infarct zone experiences significant thinning, increased wall stress, and increased regional radius of curvature. Further, regional stress increases exacerbate metabolic demand without a commensurate increase in cardiac output, leading to an energetic mismatch. Sustained abnormal stress concentrations can incite the remodeling process, leading to significant LV dilation and pump dysfunction. Once these inherent myocardial changes have occurred, they are difficult to reverse therapeutically, surgically, or otherwise. This motivates early intervention and prevention of infarct expansion.
Global single and biventricular restraint devices have attempted ventricular wall stress reduction as their mechanism of action; however, these solutions rely on oversimplified models of biomechanics, namely Laplace’s law, that ignore critical features such as wall thickness, anisotropy, and general heterogeneity of tissue properties. The lack of success in global reinforcement has motivated a local restraint approach. Though there has been a substantial interest and recent work in the development of local mechanical reinforcement to prevent infarct expansion, most studies fail to demonstrate significant improvement in ventricular function. Global single and biventricular restraint devices have attempted ventricular wall stress reduction as their mechanism of action; however, these solutions rely on oversimplified models of biomechanics, namely Laplace’s law, that ignore critical features such as wall thickness, anisotropy, and general heterogeneity of tissue properties. The lack of success in global reinforcement has motivated a local restraint approach. Though there has been a substantial interest and recent work in the development of local mechanical reinforcement to prevent infarct expansion, most studies fail to demonstrate significant improvement in ventricular function.
Local restraint development thus far has primarily been conducted through a trial-and-error approach. Recently, studies have elucidated the importance of infarction material properties on progressive pump dysfunction, encouraging the modification of infarct mechanics as an early intervention therapy. Additionally, it is typically believed that synthetic patches should match the material properties of the heart. However, the unearthing of ideal material properties and the means of achieving them through local reinforcement has yet to be achieved, in part due to the complexity of 3D regional cardiac mechanics and the time-dependent nature of the disease. With the advent of realistic multi-scale computational models, feasible solutions can conceivably be realized. The modularity of computational models allows for rapid and economical evaluation of local reinforcement parameters on quantitative, 3D regional biomechanics.
Motivated by the need for computationally optimized solutions for AMI, this research aims to take a biomechanics-based approach to the development of biomimetic local mechanical reinforcement.