Category: SummerBlogs
Week 3:
Moving some material to Private.AllisonJiaBlog for now.
Tuesday, July 6: I went to the SS lab today to work on the first phase of our plan: test current luer fitting rotation using stepper motors. I went through a couple Arduino introduction videos and learned about the stepper motor driver.
Monday, July 5: Holiday!
Week 2:
Friday, July 2: BDML had our weekly lab meeting, where lab members discussed the advantages and disadvantages of various simulation software they use. Since MDC's focus is on heart tissue (highly deformable bodies), Ali told me that we tend to use FEA software that focus more on deformations and solid mechanics rather than dynamics. After the meeting, I worked with Sam to come up with a preliminary CAD design for the catheter tip project with Grant and Avnesh. Fredrik dropped off the stepper motors for the bioreactor, and I'll be learning how to use the stepper motor driver and Arduino board throughout next week to test our motors (thank you Hojung for showing me how to solder too!).
Becky and I also got to tour the PRL today with Fred - I'm extremely excited for future classes where I'll be able to use the machines and equipment there!
Thursday, July 1: I worked with Ali and Ileana to test their heart sleeve lattice structure on a balloon and on Pinky (their soft, pink silicone model of the heart with physiological regional stiffness) since the phantom model was too stiff. Our goal was to observe whether the auxetic (negative Poisson ratio means structure expands in transverse direction when stretched) and anisotropic bowtie structure effectively restrained expansion. We soon realized that the balloon was too soft to observe any effects of the bowtie structure (air in the balloon would just inflate around the bowtie). Amy and Sam also suggested supergluing the bowtie structure to the model surface by a few corner points instead of along all the edges to allow the model to restrain global expansion instead of restraining expansion just within the bowtie area. Amy recommended using a softer glue as well to avoid creating pinch points caused by a strong glue like superglue. By the end of the day, we were able to observe that the bowtie structure was indeed acting like an auxetic material and could restrain expansion.
Wednesday, June 30: We held our third cross-lab meeting in the morning to discuss my progress and brainstorm solutions. We decided on a multi-stage plan to address the challenge of luer fitting rotation and discussed several solutions to attach the tissue to the rod.
Sam, Ali, Ileana, and I also attended a meeting with Grant and Avnesh to discuss a catheter tip design project they had asked BDML members to help CAD. Their goal is to determine the optimal geometry of side holes in a catheter tip to reduce recirculation and turbulent flow. Sam and I will work on a preliminary CAD design later in the week.
Tuesday, June 29: I met with Mark SS to go over some questions I had about the project. Fredrik and I printed our second trial version of the bioreactor using the black resin which turned out much cleaner than our first trial with the clear resin. We managed to place in 3 components: the plastic bearing, the spring-loaded plunger, and the dynamic seal. However, we soon noticed that it was extremely difficult to rotate the luer fitting, as the dynamic seal had clamped over it. I'm planning on discussing this issue tomorrow in our cross-lab meeting.
Monday, June 28: I spent the day going through DLTdv8 tutorials to help Ali and Ileana with heart tracking for their project. I learned how to do 3D tracking, digitization, and calibration, and I practiced my new skills on Ali and Ileana's earlier videos (2/24/21). I will be doing more heart tracking work on their most recent heart-sleeve pumping video (6/28/21) once I have access to the high resolution videos.
Week 1:
Friday, June 25: The lab went on our summer kickoff retreat to Butano State Park and Bean Hollow, where we wrote out and shared our summer goals! :)
Thursday, June 24: I presented my research updates at the medical devices meeting today with Mark, Ileana, and Amy. Ileana brought up several interesting questions about cardiomyocyte retention in the scaffold (which I will carry over to the SS lab). Amy recommended using teflon sheets to reduce the friction between the rod and the mold and brought up the question of whether media would be introduced into the fixed or twisting end of the tube. I attended the Skylar Scott lab meeting afterwards and learned more about the bioink that will be used to print the 3D cell tube scaffold. I plan on setting up weekly meetings with the SS team as well.
I had my last meeting with Fredrik and Sam to discuss the bioreactor model once more. We agreed to try out the components that Fredrik ordered on our first run of the bioreactor print before making new design decisions. I will also be CADing a new bioreactor design using the smaller servo motors from Sam and Tony.
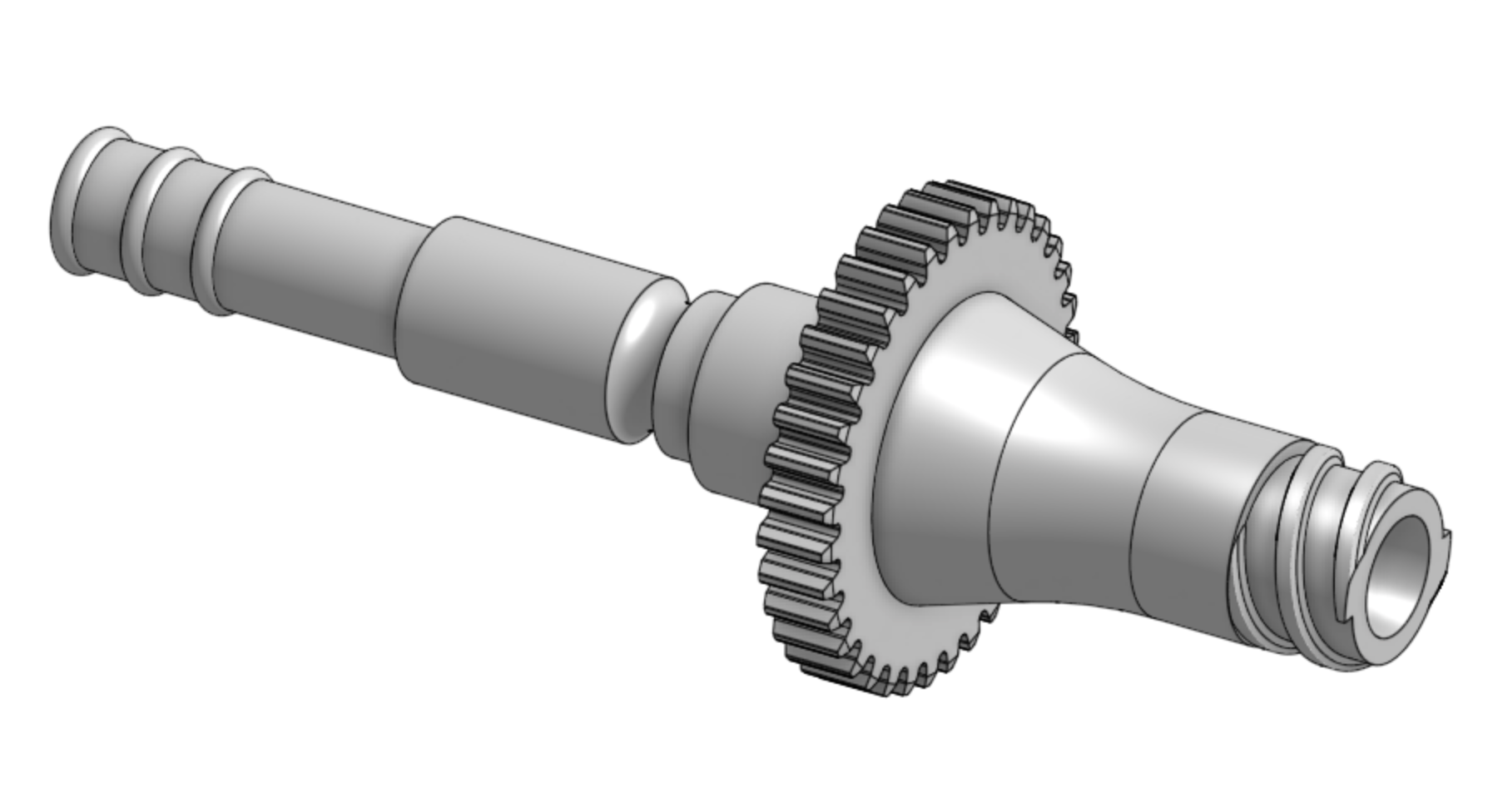
Wednesday, June 23: I spent the day in the SS lab synthesizing my own fibrin gel with Josh and running my first 3D printing trial for the bioreactor with Fredrik. I also ran my first 3D print today! Fredrik taught me how to export the Onshape design to the lab's 3D printing laptop and set up the 3D printing orientation (keep holes in the z-direction since z-direction printing has highest resolution), supports, and offset. I also learned how to work with the resin printer, set up the isopropanol wash, remove the print from the plunger/scrape off the supports using pliers/sanding the top of the print to even out the surface, air-spray dry the print, and use the UV curing device. By the end of the day, I successfully printed the luer-to-tissue shaft and part of the bioreactor mold (which could fit together)! I'll bring my prints, the fibrin gel, and one of Jessi's current bioreactor model to BDML tomorrow.
Fredrik followed up on the motor debate and brought up a point that the limited rotational motion of the servo motor may be inconvenient for tube twisting (if the tube is set up on a shaft that is already at its rotational limit, it can only rotate in one direction instead of two). I'll discuss this with Sam tomorrow.
Tuesday, June 22: Julia gave Becky and me a lab safety tour today. I then had a 2.25 hour meeting with Sam to go over the bioreactor design on Onshape and discussed motor choice, bearing placement in the mold, and design choices for the luer-to-tissue fitting.
I will be going to the SS lab tomorrow to create my own fibrin scaffold and test its material properties.
Monday, June 21: I attended the SURI kickoff today and was introduced to the other SURI interns this summer (shoutout Becky!). I came to lab in the afternoon and completed some program paperwork, read a paper on left ventricle twisting, and learned the mechanics behind torsional deformation of circular rods/tubes which will be applicable for the cell tube. I set up a meeting with Sam to discuss the Onshape bioreactor design. Dane also showed me a live demo of the gecko adhesive working to hold up a ball!
Pre-Summer:
June 16: We held our second cross-lab meeting today to discuss the project goals and design. The overarching first-pass goal of the project is to apply torque to a tube of cardiomyocyte cell tissue, disconnect the tube from the device, and observe cardiomyocyte beating and force alignment. I'll be working with Mark C, Ali, Ileana, Sam (BDML) and Mark SS, Fredrik, Jessi, and Jonathan (Skylar Scott lab) to try and integrate torque to the bioreactor. We looked over Fredrik's preliminary CAD design based on the existing bioreactor and discussed the potential components and materials needed. I'm planning to meet with Fredrik and Sam to understand and refine that design (and potentially build a physical 3D model of the bioreactor to gain a more hands-on understanding), and I'll read up on Jessi's papers about cardiomyocyte growth and development.
I'll also be assisting Ali and Ileana with marker-tracking for their heart sleeve project. I'm currently following some tutorials to learn how to use DLTdv8 in MATLAB.
May 27: I visited the Skylar Scott lab for the first time and met with Jessi, Fredrik, and Mark SS to see the bioreactor and discuss potential modification strategies. I took pictures of the bioreactor, showed the BDML members during our medical device meeting, and shared the strategies we had discussed in the SS lab.
April 27: I had my first cross-lab meeting with Mark C, Mark SS, Ali, Ileana, and Fredrik to discuss the premise of the project.
- Skylar-Scott Lab
- Link to OnShape model bioreactor with a twist
>>>>>>>